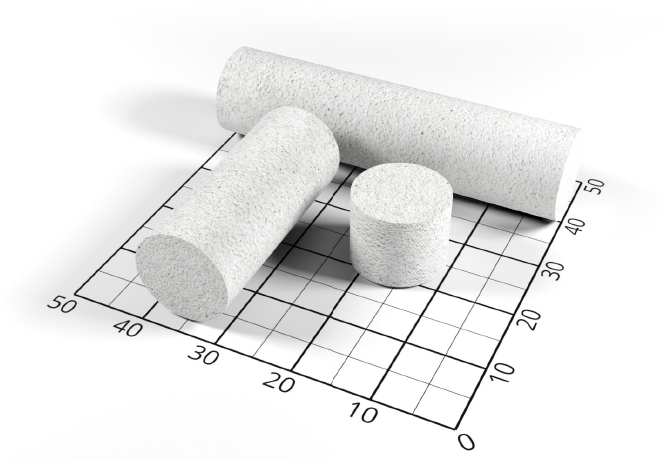
About the product
FlexiOss® Dent is a modern implantable biomaterial of third generation: Biphasic (hydroxyapatitepolymeric), bioactive, supporting the regeneration of bone tissue and a potential drug carrier.
The combination of two main compounds of composite was performed by polymer gelling into the structure of irreversible, collagen-like triple helix – the conformation which does not evoke the appearance of local inflammation processes and enables the entrapment of HAP granulate.
After implantation undergoes an integration with patient’s bone tissue in beneficial manner and is slowly remodelled and replaced with natural bone tissue. This eliminates the necessity of reoperation in order to remove the implanted biomaterial. Thus, the patient’s stress and pain is limited to the minimum.
Available in 3 sizes: 1 cm, 3 cm and 5 cm.
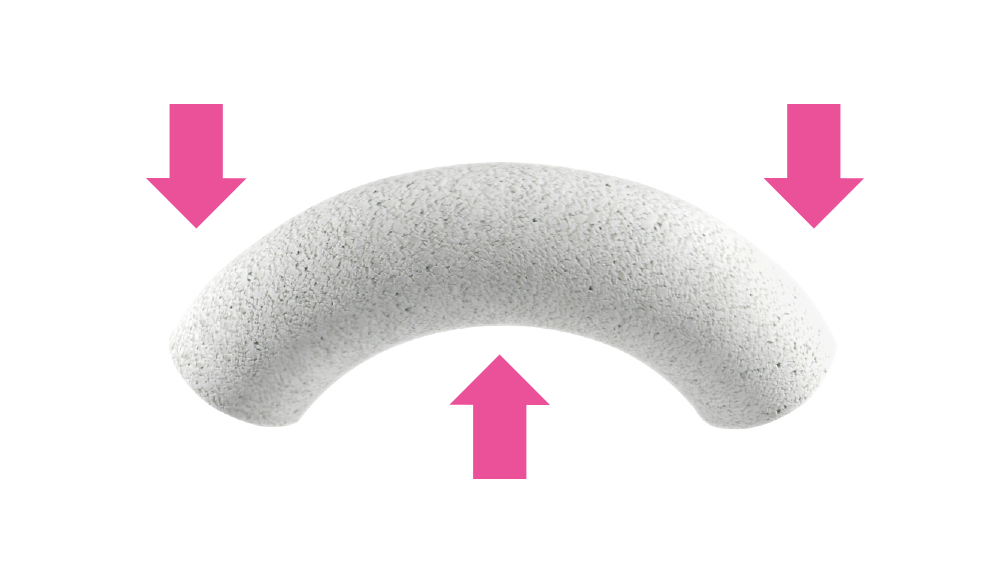
Flexible after soaking: plastic and bendable.
Properties
flexible after soaking
(plastic and bendable)
bio-compatible
with
natural bone
tissue
mechanical parameters
similar
to those
of cancellous bone
actively supports
bones
reconstruction
process
absorbs blood
and medicine
solutions
positively influences the bone tissue regeneration process
ionic reactive / bioactive
non-toxic material
can be used for
defects of
up to 7 cm in length
non-animal material (lowering risk
of pathogens transmission and appearance
of allergic reactions)
easy to carry and store


Surgical handiness
Surgical handiness, which allows to fit it during the operation to the shape of individual bone defects by cutting or bending.
Can be milled
In a dry state, FlexiOss®Dent can be milled which enables the personalization of the composite implant.
Faster ossification
High specific surface area (due to the high microporosity of granules), high ionic reactivity (adsorption of calcium and phosphate ions from surroundings liquid which allows to increase the rate of mineralization within the implantation site and increase the rate of new bone tissue formation).
Minimizes the risk of viruses and prions contamination
Application of carbohydrate polymer to produce FlexiOss® composite minimizes the risk of viruses and prions contamination. Curdlan undegoes gelling, forming the structure of irreversible, collagen-like triple helix – the conformation which is neutral for macrophages and does not evoke the appearance of local inflammation.
High soaking capacity
FlexiOss®Dent exhibit high soaking capacity. This simplify the nutrient supply to the biomaterial within the implantation site and removal of metabolic degradation products, thus increasing the rate of bone tissue regeneration. Before the implantation, it can be soaked in solution of antibacterial drugs, thus minimizing the risk of perioperative infections and implant rejection. Due to its properties, it can be also soaked in, for example, platelet rich plasma (PRP) and solution of growth factors (commercial or isolated from patient’s blood). Thus, the biomaterial may resemble (in its properties) very expensive bone-substitutes containing BMP-2-type growth factors.
Can be easily stored and transported
Can be easily stored and transported.
Studies
The biomaterial was subjected to preliminary preclinical evaluation on patients of Animal Surgery Clinic of Veterinary Medicine Faculty, University of Life Sciences in Lublin, Poland. The bone replacement material has been used for filling bone defects resulting from the teeth extraction and for treatment of oronasal fistulae in dogs and cats.
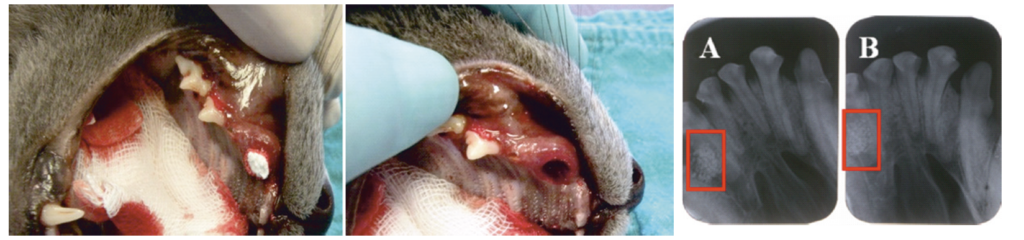
Illustration 1
The procedure of cat’s oronasal fistula filling and X-ray evaluation directly after the implantation [A] and 1 month after the implantation [B].
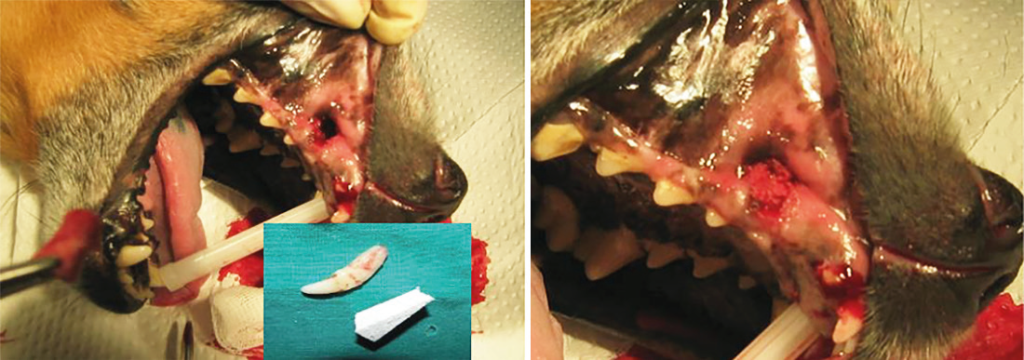
Illustration 2
The procedure of dog’s oronasal fistula filling – removal of the luxated canine tooth and filling of the fistula with biomaterial.
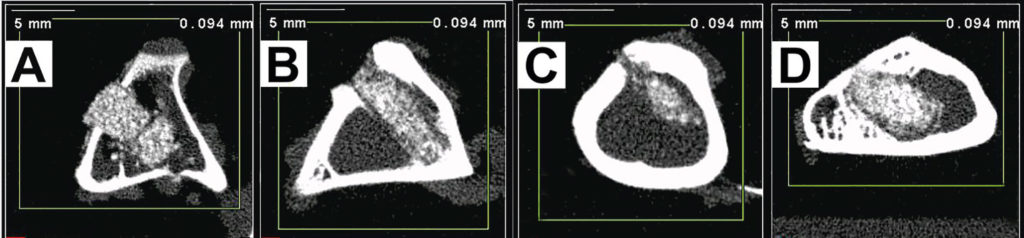
Illustration 3
Determination of bone density after the composite implantation
using microcomputing tomography technique (pQCT).
A – control image directly after the implantation (time 0),
B – image taken 1 month after the implantation,
C – image taken 3 months after the implantation,
D – image taken 6 months after the implantation.
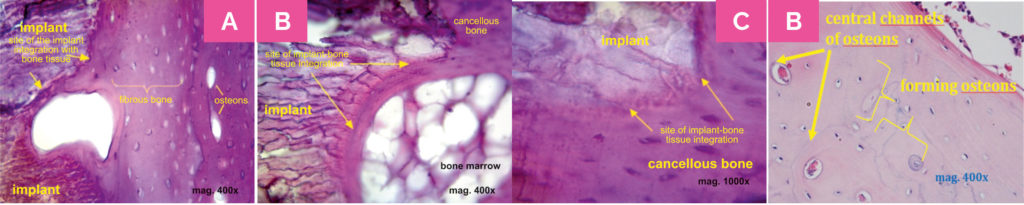
Illustration 4
Histological images suggesting integration of the implant with
surrounding bone tissue. Photography C presents the magnification
of the fragment of image B. Image D shows the bone tissue
remodelling. Histological evaluation unequivocally showed that
the process of bone tissue formation appeared in the implantations
site, which was confirmed by the presence of bone cells and
laminae, collagen fibres and Haversian canals.
Clinical observations of implanted biomaterial were conducted in patients of New-Dent Stomatological Center in Lublin, Poland. The bone replacement material showed positive effects, underwent the remodelling with the formation of bone tissue and did not evoke any side effects as the formation of bone cysts or the appearance of allergic reactions.
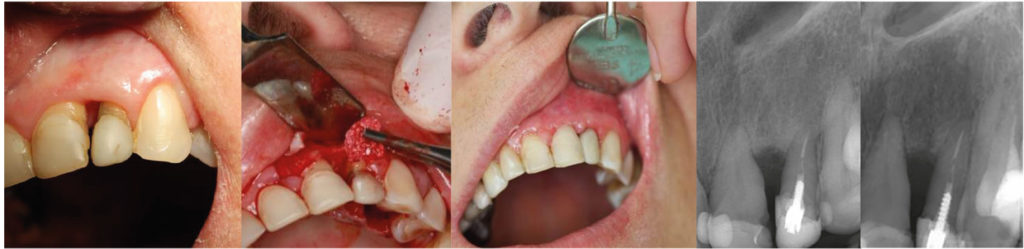
Illustration 5
Treatment of gingival resection with
serious atrophy of interdental gingiva and
bone tissue between 21st and 22nd teeth.

Illustration 6
Filling of the defect after cystotomy.
Publications
Borkowski L., Lübek T., Jojczuk M., Nogalski A., Belcarz A., Palka K., Hajnos M., Ginalska G. Behavior of new hydroxyapatite/glucan composite in human serum, Journal of Biomedical Materials Research Part B: Applied Biomaterials’ 2018 Feb 6 [Epub ahead of print].
Borkowski L., Kiernicka M., Belcarz A., Pałka K., Hajnos M., Ginalska G. Unexpected reaction of new HAp/glucan composite to environmental acidification: Defect or advantage? Biomed. Mater. Res. B Appl. Biomater. 2017, 1 05(5), 1178-1190.
Borkowski L., Sroka-Bartnicka A., Drączkowski P., Ptak A., Zięba E., Ślósarczyk A., Ginalska G. The comparison study of bioactivity between composites containing synthetic non-substituted and carbonate-substituted hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 260-267.
Belcarz A., Zima A., Ginalska G. Biphasic made of antibacterial action of aminoglycoside antibiotics- loaded elastic hydroxyapatite-glucan composite. Int. J. Pharm. 2013, 454, 285-295.
Belcarz A., Ginalska G., Pycka T., Zima A., Ślósarczyk A., Polkowska I., Paszkiewicz Z., Piekarczyk W. Application of β-1,3-glucan in production of ceramics-based elastic composite for bone repair. Centr. Eur. J. Biol. 2013, 8, 534-548.
Treatment
Sprawdź, gdzie znajduje się najbliższy punkt wykonujący zabiegi.Oława
Lublin
Warszawa
Góra Kalwaria
https://diamonddental.plSiedlce
Gdańsk
Częstochowa
Dąbrowa Górnicza
Chodzież
Contact
Medical Inventi S.A.
ul. Nałęczowska 14
20-701 Lublin
woj. lubelskie, Polska
biuro@medicalinventi.pl
NIP: 946-262-83-41
REGON: 060772285
KRS: 0000544357
Sales and Marketing
Aleksandra Machowska
aleksandra.machowska@medicalinventi.pl
tel. +48 881 750 883
Quality Department
Katarzyna Siwiec
katarzyna.siwiec@medicalinventi.pl
tel. +48 881 611 883
Olga Kruk
olga.kruk@medicalinventi.pl
tel. +48 881 550 883
Administration, EU projects
Olga Orzechowska
olga.orzechowska@medicalinventi.pl
tel. +48 667 330 883
Bank account mBank
97 1140 1094 0000 2789 8000 1001
Sąd Rejonowy Lublin-Wschód w Lublinie z siedzibą w Świdniku
VI Wydział Gospodarczy Krajowego Rejestru Sądowego
Kapitał zakładowy: 320 271,50 zł
www.medicalinventi.pl

